Introduction:
Imagine being told that your infertility might be due to a rare hormone issue – one so uncommon that only a handful of cases have ever been documented. Male FSH deficiency syndrome is exactly that: an extremely rare condition where a man’s body produces insufficient follicle-stimulating hormone (FSH), a key hormone needed for making sperm. In this friendly and informative guide, we’ll explore what this syndrome is, why it happens, how it presents in both adults and children, and what can be done about it. Whether you’re a patient looking for answers or a healthcare professional seeking detail, we’ll cover the science in a conversational tone – with compassion and clarity.
What Is Male FSH Deficiency Syndrome?
Male FSH deficiency syndrome is a form of hypogonadotropic hypogonadism that specifically involves an isolated lack of follicle-stimulating hormone (FSH) in males. In simpler terms, the pituitary gland (a pea-sized gland at the base of the brain) isn’t making enough FSH, even though it may still make other hormones normally. FSH is one of two crucial gonadotropin hormones – the other being luteinizing hormone (LH) – that control the testes. In men, FSH’s main job is to stimulate the Sertoli cells in the testes to support sperm production (spermatogenesis). LH’s job, by contrast, is to stimulate Leydig cells to produce testosterone, the hormone responsible for male puberty and male sexual traits. In male FSH deficiency syndrome, LH and testosterone levels are typically normal, so the man develops normal male characteristics and sexual function, but FSH is deficient, leading to problems with sperm production.
Another way to describe this condition is “selective FSH insufficiency”, because it’s a selective failure of the body to produce or secrete FSH. It falls under the umbrella of hypogonadotropic hypogonadism (a condition of inadequate gonadotropins), but unlike more common forms (where both LH and FSH are low, leading to low testosterone and incomplete puberty), here only FSH is abnormally low while LH is adequate. As a result, testosterone levels remain normal and virilization (male physical development) proceeds normally. However, because FSH is needed for sperm cell development, affected men often have a dramatically reduced sperm count or even zero sperm (azoospermia) in their semen.
It is important to note that male FSH deficiency syndrome is not the same as general male hypogonadism due to low testosterone. In fact, it can be thought of as a very specific kind of male infertility syndrome where the typical signs of low testosterone (like fatigue, lack of male secondary sexual features, or erectile dysfunction) are usually absent. Instead, the hallmark is normal male sexual development accompanied by infertility. Men with isolated FSH deficiency commonly appear completely normal in terms of masculinity – they go through puberty on time, have normal facial hair, muscle mass, libido, and so on – because their testosterone is normal. The problem lies “behind the scenes” in the testes: without enough FSH signal, the testes can’t efficiently produce sperm cells, leading to infertility. For this reason, male FSH deficiency syndrome is often discovered only when a man seeks evaluation for difficulty having children. It has even been described as the “mirror image” of another rare condition called the “fertile eunuch” syndrome (isolated LH deficiency), in which a man has normal FSH and can make sperm, but lacks LH and testosterone, resulting in poor virilization. In FSH deficiency, by contrast, the man is virilized and looks normal, but is infertile due to lack of sperm.
To summarize: Male FSH deficiency syndrome is a rare disorder where the pituitary gland’s failure to produce FSH leads to impaired sperm production, while testosterone levels and male development remain normal. It is one of the rare causes of male-factor infertility attributable to the hormonal (endocrine) system rather than a problem in the testes themselves or the reproductive tract. In the sections that follow, we’ll delve into what causes this syndrome, how it presents in different stages of life, how common (or uncommon) it is, and how doctors diagnose and treat it.
The Role of FSH in Male Reproduction
Understanding this syndrome is easier with a bit of background on what FSH normally does. FSH (follicle-stimulating hormone) and LH (luteinizing hormone) are gonadotropins released by the anterior pituitary gland in response to signals from the hypothalamus (GnRH, gonadotropin-releasing hormone). These hormones coordinate testicular function (see Figure 1 below). LH stimulates the Leydig cells in the testes to produce testosterone, the hormone responsible for male sexual development and traits. FSH, on the other hand, targets the Sertoli cells inside the seminiferous tubules of the testes, prompting them to aid in the formation and maturation of sperm cells. Sertoli cells, under the influence of FSH (and the local testosterone provided by Leydig cells), facilitate the entire process of spermatogenesis. They also produce substances like androgen-binding protein (to concentrate testosterone in the vicinity of developing sperm) and inhibin B, a hormone that gives feedback to the pituitary to regulate FSH levels. Inhibin B specifically travels back to the pituitary to say “we have enough FSH effect,” thereby selectively lowering FSH production when sperm production is sufficient. This fine feedback loop ensures a balance: if sperm production drops and less inhibin is made, the pituitary should ramp up FSH output; if sperm production is high, inhibin rises and signals FSH to dial back.
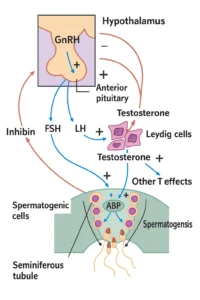
Figure 1: Simplified diagram of the male hypothalamic-pituitary-gonadal (HPG) axis. GnRH from the hypothalamus stimulates the pituitary gland to release LH and FSH. FSH acts on Sertoli cells in the testes to support spermatogenesis and to produce inhibin, while LH acts on Leydig cells to produce testosterone. Testosterone and inhibin provide negative feedback to the hypothalamus and pituitary to regulate further hormone secretion. In male FSH deficiency syndrome, the FSH signal is abnormally low or absent, impairing sperm production despite normal LH and testosterone levels.
In a normal male, this hormonal teamwork ensures both testosterone production and sperm production proceed appropriately. For example, during puberty, rising FSH levels cause the testes to grow in size (mostly by expanding the seminiferous tubules and Sertoli cell population) and kick-start sperm production. Throughout adulthood, a baseline level of FSH is required to maintain spermatogenesis. If FSH is too low (such as in certain medical conditions or due to external factors like anabolic steroid use), sperm counts will typically fall. Conversely, if the testicles themselves are not working properly to make sperm (primary testicular failure), the pituitary senses the lack of inhibin and often drives FSH levels very high in a futile attempt to stimulate the testes. Doctors often measure FSH in an infertile man as a clue: a high FSH suggests the testes are failing to produce sperm (primary testicular problem), a low FSH suggests an issue in the hormonal signaling (central or pituitary problem), and a normal FSH can indicate either normal sperm production or a blockage in the reproductive tract (because in obstructive azoospermia, sperm are made normally so FSH feedback remains normal).
Male FSH deficiency syndrome represents an unusual scenario: the testicles could produce sperm, but the pituitary isn’t sending the FSH signal. It’s as if a factory has all the machinery and workers in place (the testes and Sertoli cells), and the raw materials (testosterone, nutrients) are available, but the foreman’s instruction (FSH) is missing, so the production line never fully activates. In these men, the Leydig cells are still getting LH stimulation, so they make normal testosterone – hence normal male sexual function and puberty. But the Sertoli cells are under-stimulated, leading to either drastically reduced sperm output or none at all. The testes in such cases may be smaller than average or underdeveloped in terms of sperm-producing tissue (reflecting the lack of FSH-driven Sertoli cell proliferation). However, if LH is intact, the overall testis size might not be extremely tiny; some men with isolated FSH deficiency have nearly normal testicular volume, while others (especially those with complete congenital FSH absence) have noticeably small testes (often termed testicular hypotrophy) due to Sertoli cell underdevelopment.
As we explore this condition further, keep in mind this key concept: FSH is essential for sperm production, but not for male gender development or sexual function per se. That is why a man can appear hormonally normal in most respects and yet be infertile due to this syndrome. Next, we’ll discuss what causes such a selective hormone deficiency.
What Causes Male FSH Deficiency Syndrome?
Male FSH deficiency syndrome can arise from a few different causes, broadly categorized into congenital (genetic) causes and acquired causes. It is important to emphasize that true isolated FSH deficiency is exceptionally rare in any form. Let’s break down the known causes:
1. Congenital/Genetic Causes
Genetic mutations are the best-documented cause of true isolated FSH deficiency in males. In particular, mutations in the gene that encodes the FSH beta subunit (FSHB gene) have been identified in several cases. FSH is a protein composed of two parts: an alpha subunit (shared with other hormones) and a unique beta subunit that gives FSH its specific function. A mutation in the FSHβ subunit gene can result in the pituitary gland producing an FSH hormone that is non-functional or no FSH at all. These mutations are usually inherited in an autosomal recessive manner. That means an individual must inherit two defective copies of the FSHB gene (one from each parent) to manifest the syndrome. Typically, the parents are carriers with one normal gene and one mutated gene but no symptoms themselves.
The first reported case of a male with isolated FSH deficiency due to an FSHB gene mutation was published in 1998. This landmark case in the New England Journal of Medicine described a young man with normal puberty and testosterone, but who was azoospermic (no sperm) and had a homozygous mutation in FSHB; when doctors gave him injections of FSH (derived from human menopausal gonadotropin), he eventually was able to produce sperm and even father a child. Since that time, only a handful of additional cases with confirmed FSHB gene mutations have been documented worldwide – highlighting how rare this condition is. In 2005, a report described the clinical and hormonal features of both male and female siblings with FSHB mutations, confirming that in men it causes isolated infertility with low FSH, while in women it causes lack of ovarian development (primary amenorrhea). There have even been cases of siblings (brother and sister) both affected by a recessive FSHB mutation, as one might expect from an inherited condition. When both copies of the FSHB gene are nonfunctional, the pituitary may still release the alpha subunit or trace FSH, but essentially no effective FSH hormone reaches the testes to drive spermatogenesis.
It’s worth noting that in about half of the published male cases of isolated FSH deficiency, no mutation in the FSHB gene was found, indicating that other, yet-unknown genetic or molecular mechanisms might lead to this syndrome. Some researchers speculate that there could be mutations in regulatory regions of the FSHB gene (promoters) or in genes that control FSH release. There might also be rare cases of a developmental anomaly where the particular pituitary cells that make FSH (gonadotropes) didn’t form correctly or are selectively unresponsive to GnRH for FSH production. Another hypothetical genetic cause could involve the FSH receptor in the testes – however, a mutation in the FSH receptor (FSHR gene) would actually be a different scenario: the pituitary would make plenty of FSH (often elevated FSH levels), but the testes can’t respond to it. That condition is sometimes termed “FSH resistance” rather than FSH deficiency. Indeed, men with FSH receptor mutations can also present with infertility and small testes, but they typically have high FSH levels in blood (because the pituitary is trying hard to stimulate an unresponsive testis). So, FSH receptor problems are part of the differential diagnosis but not the same as pituitary FSH deficiency.
In summary, the primary known cause of male FSH deficiency syndrome is congenital mutation of the FSHB gene, an autosomal recessive condition. If a man is found to have this mutation, it means the condition is genetic and can run in families (siblings have a 25% chance of also being affected if both parents are carriers). Many reported genetic cases have occurred in families with some degree of consanguinity (related parents), which increases the chance of inheriting rare recessive mutations. For example, the original 1998 case was in a man of Palestinian descent with first-cousin parents, and other cases have been reported from various ethnic backgrounds including two sisters from a Kashmiri family with a homozygous nonsense mutation in FSHB. Despite these few genetic findings, the total number of known families in the world is extremely small – making it a true medical rarity.
2. Acquired or Secondary Causes
While the classic “syndrome” refers to the rare congenital form, acquired causes of low FSH levels in a man can produce a similar hormonal picture (low FSH, normal or near-normal LH and testosterone) and thus mimic FSH deficiency. It’s important for doctors to consider and rule out these more common factors before diagnosing true isolated FSH deficiency. Some of these causes include:
- Exogenous Androgens (Anabolic Steroids or Testosterone Therapy): Perhaps the most frequent cause of low FSH (and low LH) in men is the use of external testosterone or steroid hormones. When a man takes high doses of testosterone (for bodybuilding or as medication), the body’s natural feedback mechanisms detect an excess of androgens and signal the hypothalamus and pituitary to shut down GnRH, FSH, and LH production. Typically, this causes both FSH and LH to drop (not an isolated FSH drop, but a combined hypogonadotropic state). However, in some men, depending on timing and dosing, FSH might remain suppressed longer. The result is suppressed sperm production – this is essentially the mechanism behind male contraceptive attempts with testosterone. If a patient has a history of steroid use or testosterone therapy, that is usually the explanation for a low FSH, and it’s reversible by stopping the external hormones. (This scenario is not what we call “FSH deficiency syndrome” per se, but it’s a critical differential diagnosis).
- High Prolactin Levels (Hyperprolactinemia): An elevated prolactin hormone (from a prolactin-secreting pituitary tumor or certain medications) can suppress the release of GnRH from the hypothalamus, thereby reducing both FSH and LH output. In a man with a prolactinoma, often both FSH and LH are low, usually leading to low testosterone as well (causing sexual symptoms). So this usually is not an isolated FSH problem – it’s a broader pituitary issue – but it’s still an acquired cause of central hypogonadism that must be excluded. Treating the high prolactin (for example, with medications like bromocriptine or cabergoline) often restores normal gonadotropin levels.
- Pituitary or Hypothalamic Tumors or Damage: Any process that damages the pituitary gland can impair hormone production. Large pituitary tumors (adenomas) or craniopharyngiomas, for instance, can compress the cells that produce FSH/LH. Usually, such damage affects LH and FSH together (and often other pituitary hormones like TSH or ACTH as well). However, there are rare instances where a pituitary adenoma or other lesion might selectively affect certain cell populations. Some case reports describe men with pituitary tumors who had disproportionately low FSH but relatively preserved LH – possibly because the tumor or its surgery/radiation impacted some hormone cells more than others. Also, pituitary apoplexy (bleeding into the gland) or head trauma can cause partial hypopituitarism. In practice, if a man has low FSH, an MRI of the brain is often done to ensure there isn’t a tumor or structural problem. Generally, though, pituitary damage would also compromise fertility by lowering testosterone (LH) too, so isolated FSH loss is uncommon in these scenarios.
- Medications and Substance Use: Certain drugs can suppress the GnRH/LH/FSH pathway. Examples include long-term use of opioids (narcotic pain medications), which can lower gonadotropin levels, and chronic high-dose corticosteroids or severe illness/stress which can also dampen reproductive hormone secretion. Some chemotherapy or radiation to the brain could potentially affect pituitary function. Again, these often cause combined hypogonadism (low LH and FSH), not just FSH. But a thorough history of medication use is important when evaluating low FSH.
- Obesity and metabolic factors: Severe obesity and metabolic syndrome can be associated with a mild decrease in FSH and LH due to complex hormonal interactions (like elevated estradiol from fat tissue, insulin effects, etc.). However, this tends to cause subfertility via reduced testosterone and is not typically severe enough to cause isolated azoospermia. Weight loss can sometimes improve hormone levels in such cases.
- Idiopathic (Unknown) Partial Deficiency: There are men who simply have an idiopathic low FSH level without an obvious cause. In one study of infertile men, about 0.87% were found to have an isolated low FSH (with normal LH and testosterone) and no identifiable external cause. These men tended to have low sperm counts or poor sperm quality (oligoasthenoteratozoospermia – meaning low count, poor motility, and abnormal morphology of sperm) rather than complete azoospermia. It’s possible that some of these cases represent milder genetic defects in FSH production or action that are not yet understood. They might not have a total “absence” of FSH, but rather chronically lower-than-normal FSH output. This category overlaps with congenital causes in that the etiology is unknown – we just observe the phenotype of low FSH and subfertility and call it idiopathic. Ongoing research is looking at genetic variants (polymorphisms) in the FSHB gene promoter that can influence FSH levels. For instance, a particular common variant (−211G>T in the FSHB gene promoter) has been associated with slightly lower FSH levels in men and reduced sperm counts. However, carriers of such polymorphisms usually still have FSH in the low-normal range, not a near-zero FSH like the classic syndrome.
In summary, true male FSH deficiency “syndrome” most often refers to the rare congenital/genetic cases where FSH is essentially absent due to a mutation. Acquired situations (like steroid use, hyperprolactinemia, or pituitary tumors) can cause low FSH levels as well, but they usually affect LH and testosterone or have other clinical signs that distinguish them. A good clinician will evaluate for those reversible or treatable causes (e.g. checking medication history, prolactin level, MRI scan) before labeling someone with isolated FSH deficiency. Often, if those factors are present, addressing them (stopping steroids, treating the tumor, etc.) can restore fertility. But if none of those are found and a man has persistently low FSH with normal LH/testosterone, the suspicion for this rare syndrome rises.
How Does It Present? (Signs and Symptoms)
Adult Men: The typical presentation of male FSH deficiency syndrome in adults is male infertility. Most men with this condition come to medical attention because they and their partner have been unable to conceive a child. On evaluation, the man is often found to have azoospermia (no sperm in the ejaculate) or a severely low sperm count, despite having normal sexual function. This finding prompts further hormone testing, which reveals the peculiar pattern: FSH levels are abnormally low or even undetectable, while LH and testosterone levels are normal. Clinically, these men have undergone normal puberty. They generally report normal libido, normal erectile function, and can even have normal frequency of intercourse – nothing overtly suggesting a hormonal problem. They also usually have normal secondary sexual characteristics: normal muscle mass, male body hair distribution, and no breast enlargement (gynecomastia). Unlike men with typical hypogonadism, they do not have complaints of fatigue, hot flashes, or loss of libido because their testosterone is intact.
On physical examination, one subtle clue may be the testicular size. Many men with isolated FSH deficiency have smaller-than-average testes (sometimes described as testicular atrophy or hypotrophy). The testes might feel slightly soft and underfilled (because the seminiferous tubules inside, which produce sperm, are underdeveloped). In some reported cases due to FSHB mutations, testicular volume was markedly reduced – for example, testes measuring around 5–10 mL in volume (where normal adult male testes are about 15–25 mL each). However, this is not universally true. Some cases without an FSHB gene mutation have had normal-sized testes. For instance, one case report described a 22-year-old man with isolated FSH deficiency who had testes measuring 25 mL (which is normal), yet he was azoospermic. That particular patient’s condition was milder (idiopathic) and not due to an FSHB mutation, which may explain the normal testis size. In general, though, if a man has azoospermia with completely normal-sized testes, isolated FSH deficiency is only one consideration; obstructive causes or other issues would also be likely. If the testes are small, the differential shifts toward primary testicular failure – but primary failure usually raises FSH, not lowers it. So the combination of small testes + azoospermia + low FSH + normal testosterone is a red flag for this syndrome.
Men with congenital FSH deficiency do not usually exhibit any problems with sexual differentiation at birth or during puberty. Penis size and male genital development are normal, because those depend on testosterone (which is present). They also typically have normal sense of smell, distinguishing this from Kallmann syndrome (a condition of GnRH deficiency that causes both FSH and LH deficiency with anosmia). In the case series and reports, physicians often explicitly note normal virilization and normal smell testing, to underline that it’s not Kallmann. Some men might have subtle signs of long-standing FSH lack: for example, they might note that their testes never seemed to fully enlarge compared to peers, or they may have a history of undescended testes (though cryptorchidism is not a consistent feature – it usually is linked to low fetal testosterone, not low FSH). One consistent laboratory finding (aside from low FSH) in classic cases is very low or undetectable inhibin B levels, since inhibin B is produced by Sertoli cells during sperm production. In men with FSHB mutations, inhibin B tends to be extremely low (reflecting little to no spermatogenesis). Anti-Müllerian hormone (AMH), another Sertoli cell product (usually high in infants and declines after puberty when Sertoli cells mature), has been reported to be low in severe FSH deficiency cases as well. This likely indicates that Sertoli cells in those men remained in an immature state or reduced number. Interestingly, in the milder cases without FSHB mutation, inhibin B levels can be normal or even high. For example, two men described in a 2019 study had very low FSH but upper-normal to high inhibin B levels. This was paradoxical, suggesting they had functioning Sertoli cells – possibly even some spermatogenesis – which might have been enough to produce inhibin and partially suppress FSH. Those patients had severe oligospermia but not complete azoospermia, showing that the syndrome can have a spectrum of severity.
Pediatric and Adolescent Presentation: It’s rare for this condition to be identified in childhood, but understanding its potential early-life effects is useful. If a boy is born with congenital isolated FSH deficiency (e.g. due to an FSHB mutation), what signs might there be before adulthood? As noted, fetal male development is largely driven by human chorionic gonadotropin (hCG) and LH stimulating testosterone – FSH is less critical for genital development. Thus, newborn boys with isolated FSH deficiency have normal male genitalia (normal penis and scrotum) and normally descended testes in most cases. However, research and clinical observations suggest that during late fetal life and infancy, FSH does play a role in testicular growth. Male fetuses who lack FSH influence may develop smaller testes by the third trimester and at birth, because FSH is important for Sertoli cell proliferation during that period. These infants would still appear male and typical externally (since LH/testosterone were fine), but their testicular volume might be on the low side.
During the so-called “mini-puberty” of infancy (the first few months of life when gonadotropin levels transiently rise in babies), a normal boy has a surge of FSH and LH that stimulates testicular activity – in that time, Sertoli cells further multiply and produce high levels of inhibin B and AMH. A baby boy with FSH deficiency would presumably have a blunted or absent FSH surge, potentially leading to less Sertoli cell proliferation and lower inhibin B/AMH in infancy. That said, mini-puberty is not usually tested clinically unless there’s a concern like undescended testes.
As the child grows, there typically wouldn’t be obvious signs of a problem until puberty. At puberty, LH increases and should induce normal testosterone-driven changes: the boy will develop a deep voice, muscle mass, body hair, and so on. FSH’s role at puberty is to initiate sperm production and further enlarge the testes (by expanding seminiferous tubules). A teenager with isolated FSH deficiency would go through virilization normally thanks to LH and testosterone, but may have delayed or inadequate testicular growth. For example, during adolescence their testicular size might remain prepubertal or only slightly enlarged, even as penile and body hair growth occur normally. This could manifest as disproportionately small testes for their Tanner stage. An astute pediatrician or endocrinologist measuring testicular volume in a teen might notice that, say, at age 17 the patient has adult-level pubic hair and penile growth, but testicular volumes still around 4–6 mL (child-sized) instead of the expected ~15–20 mL of a mature teen. This pattern would be unusual and might prompt a hormonal evaluation. In one reported male adolescent with an FSHB mutation, the boy did show signs of selective spermatogenic failure at puberty – normal virilization but very low sperm counts and small testes.
However, because such cases are so rare and because small testes can have other causes, many boys would likely go undiagnosed until adulthood when fertility is tested. It’s also possible that some boys with partial FSH deficiency still achieve some testicular growth and even low-level sperm production, flying under the radar until they present with subfertility rather than absolute infertility.
Secondary Effects and Metabolic Impact: Outside of fertility and testicular size, does FSH deficiency cause other health issues? Generally, most bodily functions remain normal. Testosterone being normal means bone density, muscle mass, and sexual function are preserved – so these men do not have the usual metabolic or systemic problems seen in men with low testosterone (like osteoporosis, anemia, or metabolic syndrome from hypogonadism). There is some scientific interest in whether FSH itself has any direct roles in metabolism or other tissues. For example, in females, rising FSH levels in menopause have been hypothesized to contribute to bone loss and fat gain (there are experimental studies about FSH receptors in bone). In men, there is no strong evidence that low FSH by itself causes bone or metabolic disturbances. The notable “secondary” effects are mainly psychological and quality-of-life related, stemming from infertility. Men who discover they have azoospermia can experience distress, anxiety, and depression related to the diagnosis of infertility. It’s important for healthcare providers to approach this with compassion and provide counseling and support. In the context of FSH deficiency, a silver lining is that sperm production can sometimes be induced with treatment (more on that later), so there may be hope to offer.
One interesting research finding is that FSH might have a subtle facilitating effect on Leydig cell (testosterone-producing cell) function. A detailed endocrine study of a brother and sister with an FSHB mutation (the brother being the affected man) showed that giving FSH to the man seemed to enhance the response of his Leydig cells to LH/hCG. Normally, we think LH solely controls testosterone and FSH has no role in Leydig cells, but this study suggested that in the man, FSH administration increased his testosterone production above the level with LH alone. This implies a paracrine interplay: FSH acting on Sertoli cells might produce factors that help Leydig cells function optimally. Clinically, men with isolated FSH deficiency typically have normal testosterone, but that research hints that their testosterone might be just enough and that perhaps their Leydig cells are working at high LH drive. Indeed, one case noted that LH was actually somewhat elevated (though still in normal range or mildly high) in an FSH-deficient man, possibly as the body’s attempt to compensate. The elevated LH could lead to Leydig cell hyperplasia (increase in number) as was observed in some testicular biopsy samples of these patients. So in summary, while they are not overtly hypogonadal in testosterone terms, there are subtle endocrine adaptations occurring: low FSH causes low inhibin, which should cause high FSH via feedback – but since FSH can’t rise due to the defect, the hypothalamus might increase GnRH pulse frequency, preferentially boosting LH output (resulting in normal T but perhaps higher pulse frequency of LH). This could explain why some reports mention “normal or elevated LH” despite low FSH. Clinically, though, the man wouldn’t notice this; it’s just physiology trying to right the ship.
In summary, the main clinical presentation of male FSH deficiency is: a normally virilized male with unexplained infertility. Key features include azoospermia or severe oligospermia, low FSH levels on repeat testing, normal LH and testosterone levels, and often smaller testicular volume. In adolescents, a hint might be poor testicular enlargement despite normal puberty otherwise. The condition doesn’t typically cause symptoms like decreased libido or erectile dysfunction, distinguishing it from other hypogonadal states. Because fertility is the primary concern, that’s usually what drives the individual to seek medical help.
How Common Is It?
In one word: rare. Male FSH deficiency syndrome is so uncommon that each case reported in medical literature is noteworthy. It is considered a rare disease or “orphan” condition. To give a sense of the numbers:
- The prevalence in the general population is essentially unknown but extremely low. Orphanet (a database of rare diseases) lists the prevalence as unknown, reflecting how scarce data are, and confirms autosomal recessive inheritance with typical onset in adolescence or adulthood (when fertility issues become apparent).
- Among men evaluated for infertility, isolated FSH deficiency is exceptionally rare – on the order of far less than 1% of cases. One preliminary study from 2013 screened 3,335 infertile men and found only 29 who met criteria for isolated low FSH with normal other hormones, which is about 0.87%. Similarly, another review noted a retrospective analysis where about 0.89% of male infertility patients had isolated FSH deficiency. These figures suggest that even in the very select group of men who have fertility problems, fewer than 1 in 100 has this specific issue. The vast majority of male infertility is caused by other factors (genetic issues like Y-chromosome deletions, testicular varicoceles, hormonal issues involving both FSH/LH, obstructive problems, etc.). This syndrome is a tiny slice of the pie.
- In terms of documented cases in literature, by 2022 roughly a dozen individual case reports of male FSH deficiency had been published. Half of those had identifiable FSHB gene mutations, and the others were idiopathic (no mutation found). This count doesn’t include female cases (which are also rare, presenting as ovarian failure). To put this in perspective, many endocrinologists or urologists might go their whole career without encountering a confirmed case.
- The first male cases were reported only a few decades ago (1980s and 1990s), so our awareness of the condition is relatively new. One early case from 1983 described a man presumed to have “isolated FSH deficiency” based on clinical findings, and more definitive genetic-confirmed cases followed in the late 1990s. Since then, only a trickle of case reports have appeared – often each new case adds a piece to the puzzle (for example, a new mutation, or a slightly different phenotype).
- Geographic and ethnic distribution: Cases have been reported from various parts of the world – there’s no evidence of it being more common in any particular population, except that consanguineous communities might surface recessive conditions more. Known cases include individuals of Middle Eastern, European, Asian, and South American backgrounds. Since it’s autosomal recessive, it could potentially occur anywhere by chance if two carriers meet.
- Pediatric detection: As mentioned, it’s rarely diagnosed before adulthood. The “age of onset” usually listed is adolescent or adult, meaning that’s when it is recognized (even though congenital cases are present from birth, they don’t become evident until the expected time of puberty or reproduction).
To summarize: Male FSH deficiency syndrome is one of the rarest causes of male infertility, with only a handful of confirmed cases worldwide. Among infertile men, less than 1% have this specific hormonal profile. Its rarity is one reason it’s often initially overlooked and why proper diagnosis is crucial – because, as we’ll discuss next, it’s a potentially treatable form of infertility if identified. The scarcity of cases also means that our knowledge is evolving; each new case helps doctors learn more about how the condition can present and respond to therapy.
How Is It Diagnosed?

Diagnosing male FSH deficiency syndrome requires a combination of clinical suspicion, laboratory tests, and sometimes genetic analysis or imaging. Given how rare it is, a thorough evaluation is done to both establish the diagnosis and rule out other causes of the patient’s presentation. Here’s how the diagnostic process typically works:
1. Clinical Evaluation: The journey often starts in a fertility clinic or urologist’s office when a man is found to have azoospermia (no sperm in semen) on a routine semen analysis during an infertility work-up. If a semen analysis shows zero sperm or a very low count, doctors will take a detailed history and physical exam. Key points that raise suspicion for hormonal issues include: history of delayed puberty or issues with sexual function (usually absent in isolated FSH deficiency), any use of testosterone or steroids (which could explain low FSH), previous fertility, and any symptoms of pituitary problems (like headaches or vision changes that might suggest a tumor – again usually not the case here). On exam, testis size will be assessed (often with an orchidometer or ultrasound). In isolated FSH deficiency, the findings may be normal virilization with possibly smaller testes as discussed. The presence of a very small testicular volume and azoospermia might initially make the clinician think of primary testicular failure (like Klinefelter syndrome), but in that case FSH would be high, not low. So the next step is crucial:
2. Hormone Testing: The cornerstone of diagnosis is a hormonal blood test panel. This typically includes: FSH, LH, testosterone, and often prolactin and estradiol (to check for other imbalances). In male FSH deficiency syndrome, the lab results characteristically show: low FSH, normal LH, and normal testosterone. Sometimes LH can be at the high end of normal or slightly elevated, but not inappropriately low; testosterone should be in the normal adult male range (often mid-normal, since LH is working adequately). Prolactin is typically normal (unless there’s an unrelated issue). Estradiol is usually normal; a very high estradiol with low FSH might hint at an estrogen-secreting tumor or obesity-related changes rather than isolated FSH deficiency. Thyroid function is also sometimes checked, as severe hypothyroidism can raise prolactin and affect gonadotropins, though that scenario usually raises FSH, not lowers it.
One specialized test that can be done is a GnRH stimulation test (injecting GnRH and measuring subsequent FSH/LH response). In normal individuals, GnRH will cause both LH and FSH to rise. In men with isolated FSH deficiency of pituitary origin, giving GnRH tends to result in a blunted or absent FSH response, while LH responds vigorously. For example, in the case from 2022, an IV GnRH injection caused LH to rise appropriately, but FSH remained low, confirming the pituitary’s selective inability to secrete FSH. This test isn’t done in every case but can help differentiate between a hypothalamic cause (where maybe GnRH pulses are skewed) versus a pituitary issue; however, in an FSHB mutation scenario, even flooding with GnRH can’t overcome the lack of a functional FSH beta subunit, so FSH stays low.
Another useful marker is inhibin B. As noted earlier, inhibin B is secreted by Sertoli cells when active spermatogenesis is occurring. In a man with azoospermia, a very low inhibin B suggests severe Sertoli cell dysfunction or absence of germ cells. In classic FSH deficiency (like FSHB mutation), inhibin B is very low, consistent with lack of sperm production. Some clinicians will measure inhibin B to help confirm that the low FSH is truly associated with low Sertoli cell activity (and not a lab error or something). In the atypical cases where inhibin B is not low, one must reconsider if there is some degree of spermatogenesis occurring or an alternate explanation for low FSH.
3. Differential Diagnosis: Once the lab pattern of low FSH, normal LH, normal T is established, it’s crucial to rule out other conditions that could cause a similar pattern:
- Could it be just prior testosterone use? A detailed history is needed. If the patient has been on anabolic steroids or testosterone supplements, that could cause low FSH and LH. In such cases, typically both FSH and LH are suppressed (often profoundly so), and testosterone might be high (if still on therapy) or low (if recently withdrawn and endogenous system suppressed). Isolated FSH suppression with normal LH is not a typical finding in exogenous androgen use – so a normal LH level makes significant external androgen exposure less likely (or it’s been long enough since use that LH recovered but FSH didn’t – again unusual). Nonetheless, the physician should explicitly ask about and rule out any exogenous hormone use or other drugs that affect the pituitary.
- Could it be a mild case of Kallmann syndrome or GnRH deficiency? In congenital GnRH deficiency (idiopathic hypogonadotropic hypogonadism), usually both LH and FSH are low and testosterone is low, leading to incomplete puberty – clearly a different picture. However, there are rare mild cases of GnRH neuron dysfunction where the balance of LH vs FSH secretion could be uneven. One condition called “fertile eunuch” (a variant of isolated GnRH deficiency) results in low LH (hence low testosterone and poor virilization) but relatively preserved FSH, allowing some sperm production. That’s essentially the opposite of isolated FSH deficiency. So confusing the two is not likely – their clinical presentations are very different (one is under-virilized with low T, the other is normally virilized with normal T). Anosmia (loss of smell) would point to Kallmann syndrome, but in our case the sense of smell is normal.
- Could it be primary testicular failure with a lab error? If a man had Sertoli-cell-only syndrome (a condition where the testes lack germ cells), he would be azoospermic and typically have high FSH (due to lack of inhibin) but normal LH and normal testosterone (Leydig cells intact). That scenario yields a high FSH, not low, so it’s basically the inverse hormone pattern of FSH deficiency. There is little chance to mistake one for the other if labs are done accurately. If a lab report showed low FSH but the clinical picture looked like primary testis failure, one might even consider repeating the test to exclude lab error. Labs measuring FSH generally have good accuracy, but very low levels close to the detection limit can sometimes be tricky. Therefore, physicians will usually recheck hormone levels for confirmation before pronouncing a diagnosis.
- Chromosomal abnormalities: Klinefelter syndrome (47,XXY) is a common cause of azoospermia with small testes, but it features high FSH and high LH with low testosterone typically. So that doesn’t fit. Other genetic syndromes affecting testes (e.g. DAZ gene deletions, Y chromosome microdeletions) again cause high FSH due to testicular failure. So low FSH effectively rules out most primary testicular etiologies of azoospermia. A karyotype or Y-deletion test might still be done in an azoospermic man as routine, but if FSH is low, those tests are expected to be normal (and indeed in reported FSH deficiency cases, karyotypes have been normal 46,XY males).
- Imaging: To evaluate the pituitary, an MRI of the brain is often ordered. In isolated FSH deficiency syndrome, pituitary imaging is typically normal (no tumor or structural abnormality). This was seen in reported cases – MRI showed no pituitary tumor or lesion. Finding a normal MRI helps rule out a mass lesion as the cause. If a pituitary adenoma were found, the scenario might shift – for example, a certain kind of pituitary tumor called a “gonadotroph adenoma” can paradoxically secrete FSH or the alpha subunit and sometimes cause high FSH; or a non-functioning adenoma can cause partial hypopituitarism. But these do not selectively knock out FSH without affecting LH in most instances. A normal MRI and normal other pituitary hormones strongly suggest a genetic or idiopathic cause.
4. Genetic Testing: If isolated FSH deficiency is strongly suspected (particularly in a young man with life-long infertility and very low FSH), genetic testing for FSHB gene mutations can be pursued. This is usually done via sequencing the FSHB gene (which is a relatively small gene). A clinical genetic test might be available through specialized labs or research settings, as this is not a common commercial test. In several published cases, sequencing of FSHB was performed and found homozygous mutations. In the 2022 Greek case, they sequenced the FSHB gene but did not find a mutation, indicating an idiopathic case. If a mutation is found, it clinches the diagnosis of genetic isolated FSH deficiency – and also has implications for family counseling (parents as carriers, etc.). If no mutation is found, the diagnosis can still stand based on the clinical picture, but it will be considered idiopathic unless future research identifies another gene or mechanism. For example, one might consider research testing for mutations in the GnRH receptor gene or other pituitary transcription factors, but those usually cause combined LH/FSH deficiencies, so they are less likely.
5. Testicular Biopsy (in some cases): Occasionally, to further understand the state of the testes, a testicular biopsy is done. While not required to diagnose FSH deficiency, biopsy findings can be quite illustrative. In reported cases, testicular histology often shows an arrest of spermatogenesis – meaning the early germ cells may be present but they don’t develop into mature sperm. Frequently there is a Sertoli cell-only pattern or Sertoli cell hypoplasia, reflecting that without FSH the Sertoli cells are few and not supporting germ cells. Leydig cell hyperplasia (increased Leydig cells) has also been noted, presumably because LH is normal or elevated and continues to act on Leydig cells even though not as much sperm is being made. One case without FSHB mutation had a detailed biopsy: it showed spermatogonia present but maturation arrest, and interestingly confirmed there was no immunohistochemical evidence of FSH in pituitary tissue, supporting a true FSH deficiency not due to receptor problem. Biopsy is invasive, so it’s not routine, but if someone is undergoing exploratory testicular sperm extraction (TESE) for fertility or diagnostic purposes, the tissue can provide these insights.
Putting it all together: The diagnosis of male FSH deficiency syndrome is made when a man has (a) infertility due to azoospermia or severe oligospermia, (b) persistently low FSH levels on blood tests, (c) normal or compensatorily elevated LH with normal testosterone levels, and (d) exclusion of other causes like anabolic steroid use, hyperprolactinemia, or a pituitary tumor (via history, labs, imaging). Genetic confirmation via an FSHB gene test can solidify the diagnosis if positive.
For example, consider a scenario: a 30-year-old man with 2 years of infertility, semen analyses showing azoospermia. He has normal erectile function and no past steroid use. Physical exam: normal male body habitus, each testis ~8 mL (slightly small). Lab results: FSH <0.5 mIU/mL (low), LH 6 mIU/mL (normal), total testosterone 600 ng/dL (normal), prolactin normal. MRI of the head: normal pituitary. This profile fits isolated FSH deficiency. The physician might then order FSHB gene sequencing – if it finds, say, a homozygous nonsense mutation, that confirms a genetic case. If it finds nothing, we term it idiopathic isolated FSH deficiency. Either way, the man would be counseled that his infertility is due to lack of FSH stimulation and that treatment with FSH hormone might be needed to have biological children.
It’s also worth mentioning that diagnosing this syndrome often requires a high index of suspicion. Many infertile men won’t have hormone levels checked beyond a single total testosterone, especially if they appear virilized – but in an azoospermic man, guidelines do recommend checking FSH and LH to differentiate obstructive vs non-obstructive azoospermia. If that is done, most azoospermic men will have either high FSH (testicular failure) or normal FSH (obstruction or mild cases). A low FSH will jump out as unusual, prompting deeper investigation. Thus, awareness of this condition among fertility specialists is important so that such a clue is not missed.
Diagnostic Summary: The “classic” lab signature of male FSH deficiency is isolated low FSH with otherwise normal male hormonal profile. Confirmatory tests can include GnRH stimulation showing poor FSH release, and possibly genetic tests for FSHB mutation. Careful exclusion of other causes is part of the work-up. Once diagnosed, attention turns to management and treatment options, which we will discuss next.
How Is It Treated?
The good news for men with isolated FSH deficiency is that treatment is available and can often restore fertility or significantly improve sperm production. Since the fundamental problem is a hormone deficiency, the treatment essentially involves replacing or mimicking FSH. However, because this condition is so rare, there is no one-size-fits-all protocol – much of the knowledge comes from individual case reports and small series. Here are the main approaches to treatment:
1. Exogenous Gonadotropin Therapy (FSH Replacement): The most direct treatment is to give the missing hormone – FSH – through injections. In practice, this is usually done using either human menopausal gonadotropin (hMG) or recombinant FSH preparations. HMG (also known as menotropin) is a medication derived from the urine of postmenopausal women that contains both FSH and LH activity. Recombinant FSH (rFSH) is a lab-made FSH identical to the human hormone. Both have been used in reported cases. The goal is to stimulate the Sertoli cells and jump-start spermatogenesis.
- In men with congenital FSH deficiency due to FSHB mutation, giving FSH can have remarkable results. For example, in the earliest documented case (FSHB mutation), treatment with hMG (which provided the FSH the man lacked, plus some LH support) led to the appearance of sperm in the ejaculate after some months, and ultimately the man’s wife conceived. More recently, a 2022 case report in Cureus described a 28-year-old man with suspected isolated FSH deficiency who was treated with hMG injections three times a week; after 5 months, his sperm count improved from zero to 6 million/mL, and motile sperm were retrieved for IVF, resulting in a successful pregnancy. In the 2019 French cases without FSHB mutations, they gave exogenous FSH which led to one man achieving a spontaneous pregnancy with his partner and the other requiring IVF (ICSI) to conceive.
- Typically, the regimen might involve regular FSH injections for many months, because it takes about 72-90 days for a full cycle of spermatogenesis. Patience is key – results are not immediate. Doctors often monitor progress via periodic semen analyses to see if sperm are appearing, and sometimes by measuring testicular volume or inhibin B levels. Indeed, there are reports that exogenous FSH therapy increases testicular volume (essentially “growing” the testes by stimulating Sertoli cell activity). In one discussion, after a course of FSH injections, men had a noticeable testis size increase and resumption of sperm production, and in some cases pregnancies were achieved naturally or through assisted means. This is quite gratifying, as it indicates that the testes were fundamentally capable of making sperm – they just needed the hormonal push.
- Duration of therapy depends on response. Some men might see sperm in ejaculate after 3-4 months; others might take a year or more of therapy to optimize the chances. If the partner is of advanced maternal age or time is of the essence, often assisted reproductive techniques (like IVF) are used as soon as any sperm can be obtained (even if just a few from a testicular biopsy or ejaculation).
- LH supplementation: In true isolated FSH deficiency, LH is endogenous and normal, so giving extra LH (like hCG injections) may not be strictly necessary for spermatogenesis – since the man’s Leydig cells are already making testosterone. However, in practice, many protocols for male infertility use a combination of FSH and hCG to mimic the natural stimulation. For example, some clinicians might give hCG injections first (to ensure intratesticular testosterone is adequate) and then add FSH. In a man with normal testosterone, hCG might not be needed, but low-dose hCG could be given to ensure the intra-testicular testosterone environment is optimal for FSH to work. In some reported cases, only FSH was given and it worked; in others, hCG (which acts like LH) was combined. The 2008 sibling case study explored giving hCG, FSH, or both to see effects on sex steroids. They found that adding FSH in the presence of hCG boosted some steroid levels in the man, supporting that combination can be synergistic. Practically, though, if LH and testosterone are normal, the key missing ingredient is FSH.
- Success rates: Because the number of treated patients is small, we don’t have a percentage success like we do for more common conditions. But from the literature: about half a dozen men have been treated and several achieved pregnancies (some naturally, some via IVF). In general, this is considered a treatable cause of infertility, which is why recognizing it is important. In contrast to some other causes of non-obstructive azoospermia where it’s very difficult to get sperm, here we are limited by the hormone – and if we replace it, the testes often respond.
- Side effects and monitoring: FSH injections are generally well tolerated. Unlike giving a man LH (hCG), which can raise testosterone and potentially cause symptoms like acne or mood changes, pure FSH won’t drastically change how he feels in terms of libido or energy because his testosterone is already normal. Some men may experience enlargement of testes (which is actually desired), and maybe mild fluid retention or injection-site reactions. There is a theoretical risk of overstimulation – e.g., if the man had any tendency toward testicular tumor, stimulating Sertoli cells could conceivably influence that, but no such issues have been reported in these patients. Monitoring would include periodic hormone tests and semen analysis. If inhibin B starts rising, that’s a good sign spermatogenesis is picking up.
It is also worth noting that if fertility is achieved or the couple completes childbearing, one can stop FSH therapy. Since it’s not needed for health outside of fertility, the man can function normally without it (just infertile). Some patients might choose to bank sperm after treatment for future use, so they don’t have to stay on long-term therapy.
2. Treating Underlying Causes (if any): If an acquired cause was identified, obviously addressing that is primary. For example:
- If the man had been on anabolic steroids, the first step is stopping those. Often, testicular function may gradually recover on its own over months. Sometimes medications like clomiphene citrate or hCG are given to help kick-start the pituitary and testis recovery post-steroid use. Those drugs wouldn’t specifically address “FSH deficiency syndrome” since that’s a distinct scenario, but it’s part of the differential management.
- If a pituitary tumor were found (which in isolated FSH deficiency it typically is not), treating the tumor (surgery or medications for prolactinoma, etc.) might restore normal FSH/LH production. For example, if a man had mild hyperprolactinemia suppressing FSH, a dopamine agonist could normalize prolactin and thereby allow FSH to rise, potentially restoring fertility.
- In idiopathic cases where no reversible factor is found and no genetic mutation either, some doctors have tried using selective estrogen receptor modulators (SERMs) like clomiphene to boost gonadotropins. Clomiphene blocks estrogen feedback at the hypothalamus/pituitary, often raising both FSH and LH. In a true isolated pituitary defect, SERMs might not help if the pituitary cells cannot make FSH. But if the problem was a subtle hypothalamic imbalance or partially low GnRH drive, SERMs could potentially increase FSH. For instance, in mild idiopathic cases (say FSH is low-normal rather than completely absent), a trial of clomiphene might raise FSH a bit and improve sperm parameters. There isn’t specific literature on clomiphene for confirmed isolated FSH deficiency, but it’s a consideration in the broader context of treating unexplained oligospermia with low-normal gonadotropins. At least one study indicated that if no reversible causes are found for low FSH, SERMs or even aromatase inhibitors can be used to stimulate the pituitary to make more FSH. However, in congenital FSHB mutation cases, this won’t work – you need to give actual FSH.
3. Assisted Reproductive Technologies (ART): If hormone therapy alone doesn’t lead to pregnancy the natural way, couples can turn to ART for help:
- Intrauterine insemination (IUI): If FSH therapy results in some sperm in the ejaculate but the count/motility are low, doctors might concentrate the sperm and perform IUI to improve chances.
- In Vitro Fertilization (IVF) with Intracytoplasmic Sperm Injection (ICSI): This is often utilized if sperm counts remain very low. In ICSI, a single sperm is injected directly into an egg. In cases of FSH deficiency, even if only a few sperm appear in the semen or can be retrieved from the testes, ICSI can use them to fertilize eggs. Many of the reported successful pregnancies in these couples were via IVF/ICSI, which can circumvent the need for obtaining high numbers of sperm.
- Microsurgical Sperm Extraction: If after some treatment no sperm reach the ejaculate, sometimes a testicular sperm extraction (TESE) is done to see if any pockets of spermatogenesis are present in the testes. If found, those sperm can be used for ICSI. Because FSH deficiency could theoretically result in patchy areas of spermatogenesis (maybe some tubules started producing sperm while others not), TESE could be considered. However, the preference is to try medical therapy first to globally stimulate the testes.
4. Supportive and Psychological Care: Dealing with infertility can be challenging for a man’s mental health. Part of compassionate care is ensuring patients have access to counseling and support groups if needed. It’s important to communicate that having this syndrome is not the man’s “fault” – often men feel guilty or inadequate when an infertility factor is identified in them. Understanding the condition – that it is a rare biological quirk often due to a genetic chance – can sometimes help alleviate self-blame. We as healthcare providers should reassure patients that effective treatments exist and many men with this diagnosis have successfully had children, which can provide hope.
5. Treating Children/Adolescents: In a pediatric scenario (which is hypothetical since usually it’s not diagnosed until fertility is an issue), one might consider interventions during puberty. If a teenage boy was known (perhaps due to an affected sibling or genetic test) to have isolated FSH deficiency, a doctor could potentially give FSH in puberty to ensure normal testicular development. There isn’t documented practice of this because so few cases are known at that stage. But by analogy to treating other forms of hypogonadotropic hypogonadism, one could introduce low-dose FSH alongside hCG during puberty to promote testicular growth and preserve fertility prospects. For instance, in Kallmann syndrome (GnRH deficiency), pediatric endocrinologists sometimes give hCG (as LH substitute) for virilization and later add FSH to induce sperm when appropriate. In isolated FSH deficiency, LH and T are fine, so virilization would happen on its own; adding FSH in late puberty might help achieve a more normal testis size and possibly allow spermatogenesis to start, avoiding long-term azoospermia. This is a theoretical approach – given how rare the condition is, formal recommendations don’t exist, but it’s something a specialist might consider in the unique event of early diagnosis.
Is treatment always required? For general health, a man with isolated FSH deficiency does not need treatment because his testosterone is normal. The primary indication for treatment is fertility. If, for example, a man with this syndrome does not desire children, he might opt not to undergo any therapy. He would have to accept that he will likely not father biological children without treatment, but otherwise he can live a healthy life. Some individuals might also consider donor sperm for achieving pregnancy, if they prefer not to go through medical treatment or if treatment fails. This is a personal decision and can depend on factors like cost of therapy, success chances, and the couple’s feelings about using donor gametes. It’s our role to discuss all options openly and empathetically.
Family Planning and Genetic Counseling: If the condition is confirmed to be genetic (FSHB mutation), genetic counseling is advised. The man’s siblings might be informed of the possibility (especially any brothers about fertility, or sisters about reproductive health since female carriers might have reduced fertility if partially affected). If the couple undergoing treatment has a successful pregnancy, the children will be carriers at minimum (since the father has two mutated genes, he passes one to each child; the mother presumably is not a carrier in most cases unless it was consanguineous). If they have a son and the mother happened to be a carrier (e.g. if the parents were related), then the child could actually have the condition too (25% chance). But typically, in sporadic cases, the mother is not a carrier, so children would just carry one mutated gene (and are unlikely to have issues, though a daughter carrying one might have slightly lower fertility by some reports of female carriers – e.g., slight delays in puberty or lower ovarian reserve, as heterozygous effects). This might be an area of further study. At any rate, knowledge of the genetic nature can empower family members to get tested if needed and plan accordingly.
Follow-up: If fertility is achieved or attempted, follow-up includes monitoring hormone levels if on therapy, checking for return of azoospermia after stopping therapy (it likely will revert if FSH shots are stopped), and managing any pregnancy outcomes. Long-term, men with treated FSH deficiency do not have special health problems aside from infertility. They might be advised to store frozen sperm when it’s obtained, as a backup for future attempts.
In summary, treatment for male FSH deficiency syndrome is focused on fertility restoration via hormone therapy with FSH, often successfully leading to sperm production and even natural conception or conception through IVF. Addressing any underlying suppressive factors and providing psychosocial support are also important. It’s a story where identifying the correct diagnosis – though rare – opens the door to a therapy that can change a patient’s life by enabling them to have children. Not every cause of male infertility is so amenable to treatment, which makes this syndrome particularly rewarding to treat when recognized.
Is It Genetic or Familial?
As discussed under causes, many cases of true isolated FSH deficiency are genetic. The primary genetic cause identified is a mutation in the FSHB gene, inherited in an autosomal recessive pattern. So yes, in those cases it is a genetic condition and can run in families.
If a man has an FSHB gene mutation (homozygous or compound heterozygous), each of his parents is likely an unaffected carrier. Siblings of that man have a 25% chance of also having the syndrome if both parents carry the same mutation, a 50% chance of being carriers, and 25% chance of inheriting neither mutated gene. There have indeed been familial occurrences: for example, two sisters both with FSHB mutations causing isolated FSH deficiency (in females this leads to lack of puberty and infertility), and a report of a brother and sister both affected (the brother with our discussed male syndrome, the sister with ovarian failure). Another published family from Brazil had a brother and sister with the same mutation Tyr76X in FSHB, confirming the recessive inheritance. These cases are rare, but they reinforce that when we find one person with this condition, it’s worth checking siblings if they have any infertility or pubertal issues.
For a man diagnosed with isolated FSH deficiency, genetic counseling is advisable. A genetic counselor or knowledgeable physician can explain the inheritance. If the man’s partner is a relative (consanguineous marriage), there could be a concern that she might also carry a mutation in FSHB (if from the same family lineage). If the female partner were a carrier, then each child could potentially inherit two bad copies – meaning a son would also have the syndrome or a daughter would have ovarian FSH deficiency. However, this scenario is unlikely unless they are blood relatives or from a population with a founder mutation.
If the cause of FSH deficiency in the man is not an FSHB mutation (like those idiopathic cases), then by definition we don’t have a known genetic marker. It could still be genetic in some other way – but until identified, we can’t test family members for it. We might assume those are sporadic or less likely to cluster in a family, though we cannot be sure. The half of cases without an FSHB mutation did not report a family history of similar issues, suggesting those might be isolated events or different mechanisms.
Familial fertility implications: If a man with FSHB mutation has children (through treatment), all his children will receive one copy of the mutated gene from him. If his partner is not a carrier, the children will each carry one mutated gene and one normal gene. Carriers (with one mutation) are typically asymptomatic, but there is some curiosity in the literature if female carriers have any subtle effects (perhaps a slightly smaller ovarian reserve). Thus far, carriers have essentially normal fertility, as the presence of one normal FSHB gene is sufficient to produce FSH. So the risk to children is minimal unless they were to coincidentally partner with another carrier down the line.
Differentiating from other familial conditions: When a physician encounters a family with multiple infertile individuals, they might consider other genetic syndromes too. For example, there are families with hypogonadotropic hypogonadism due to GnRH receptor mutations or Kallmann syndrome with X-linked inheritance – but those usually involve LH and FSH both. Families with just selective FSH issues are exceedingly rare, again highlighting that an FSHB gene defect (recessive) is one clear mechanism.
In summary, male FSH deficiency syndrome often has a genetic basis (autosomal recessive FSHB mutation) and thus can be familial in those cases. If a specific mutation is found in a patient, testing his siblings or at least being aware of their reproductive health is prudent, because sisters could have ovarian issues and brothers could have similar infertility. If no mutation is found, a genetic cause is less certain, and the condition in that individual might be sporadic. Either way, once it’s diagnosed, informing the patient about the potential genetic aspect is part of good care.
It’s also worth saying that even though it’s genetic in many cases, it is so rare that it generally doesn’t run in multiple generations frequently – we don’t see a pedigree of many affected males because they usually wouldn’t have kids to pass it on (unless treated). Instead, it might manifest as an isolated instance in a generation due to two carriers meeting. Thus, families typically have at most a couple of siblings affected, rather than, say, an affected father and son (that would require a fairly unlikely scenario of the father being treated and having a son who by chance also got both mutated genes).
A Comparison with Other Male Infertility Conditions (Table)
For clarity, especially for healthcare professionals, the following table contrasts isolated FSH deficiency with some other causes of male infertility in terms of hormone profiles and clinical features:
Table Note: The above comparisons illustrate how isolated FSH deficiency stands out by having low FSH but otherwise normal hormonal milieu, unlike primary testicular failure (high FSH, high LH) or total hypogonadotropic hypogonadism (low FSH, low LH, low T). This can aid physicians in pinpointing the cause of azoospermia. Patients can also see from this comparison that their condition is quite unique among causes of male infertility.
Conclusion
In the end, male FSH deficiency syndrome is a fascinating and rare endocrine disorder – one that underscores the intricacies of human reproduction. For patients, learning about this condition can be empowering: if you’ve been diagnosed with it, you now know that you are not alone (even though it’s very uncommon) and that effective treatments exist to help you potentially achieve fatherhood. It’s normal to feel overwhelmed or even isolated when told you have a “one in a million” diagnosis, but rest assured that medical science has identified the issue and there are documented successes of men with this condition having children after appropriate therapy. The journey may require patience, hormone injections, and coordination with fertility specialists, but the possibility of fulfilling your dream of a family is real. Additionally, aside from fertility hurdles, this syndrome does not typically impose other health limitations – you can lead a healthy life with normal masculinity and sexuality, which is an important reassurance.
For healthcare professionals, male FSH deficiency syndrome serves as a reminder to always check the details: an azoospermic man with low FSH should prompt careful evaluation for this rare entity. By recognizing the tell-tale signs – isolated low FSH, normal testosterone, small testes – you can make a pivotal difference in a couple’s life by guiding them to the correct treatment. It also highlights the value of personalized medicine; because this condition is often genetic, a precise molecular diagnosis (like finding an FSHB mutation) can direct therapy and counseling. It’s a condition where endocrinology and reproductive medicine beautifully intersect, demonstrating how replacing a missing hormone can restore a physiological function as profound as creating new life.
We hope this overview has shed light on what male FSH deficiency syndrome is, what causes it, how it presents across the lifespan, how exceedingly rare it is, how we diagnose it, and how it can be treated. If you or someone you know is navigating this condition, remember that open communication with your doctors, staying informed, and seeking support are key steps. Modern medicine, through hormone therapy and assisted reproduction, offers a pathway to overcome the infertility caused by this syndrome in many instances – turning a once insurmountable obstacle into a challenge that can be managed and often overcome.
In conclusion, male FSH deficiency syndrome may be rare, but it is understandable, diagnosable, and treatable. With the collaboration of endocrinologists, urologists, reproductive specialists, and geneticists – and the courage and perseverance of patients – families have been and will continue to be built despite this uncommon diagnosis. Every successful case is not only a victory for one family but also adds to the medical community’s knowledge, hopefully making the road smoother for the next patient. If you are that next patient reading this: take heart, ask questions, and know that you have a team of professionals and the experience of those who came before you to guide you on your journey to parenthood.
References:
- Vogiatzi E, Psachna S, Ioannidis D, et al. Isolated FSH deficiency. A rare cause of male infertility. Endocrine Abstracts. 2022;81:EP914.
- Salama N, El-Sawy M. Isolated low follicle stimulating hormone (FSH) in infertile males – a preliminary report.Arch Ital Urol Androl. 2013;85(3):124-128.
- Mantovani G, Borgato S, Beck-Peccoz P, et al. Isolated follicle-stimulating hormone (FSH) deficiency in a young man with normal virilization who did not have mutations in the FSHβ gene. Fertil Steril. 2003;79(3):573-576.
- Medscape. Jabbour SA. Follicle-Stimulating Hormone Abnormalities – Overview & Practice Essentials. Updated June 09, 2023.
- Lofrano-Porto A, Casulari LA, Nascimento PP, et al. Effects of follicle-stimulating hormone and human chorionic gonadotropin on gonadal steroidogenesis in two siblings with an FSH beta subunit mutation. Fertil Steril. 2008;90(4):1169-1174.
- Phillip M, Arbelle JE, Segev Y, Parvari R. Male hypogonadism due to a mutation in the gene for the beta subunit of follicle-stimulating hormone. N Engl J Med. 1998;338(24):1729-1732.
- Berger K, et al. Clinical and hormonal features of selective FSH deficiency due to FSHβ gene mutations in both sexes. Fertil Steril. 2005;83(2):466-470.
- Frontiers in Endocrinology. Young J, et al. Spreading the Clinical Window for Diagnosing Fetal-Onset Hypogonadism in Boys. Front Endocrinol (Lausanne). 2014;5:51.
- Cleveland Clinic. Follicle-Stimulating Hormone (FSH): What It Is & Function. (Accessed Nov 2025).
- Ratnayake GM, Weerathunga PN, Ruwanpura LP, et al. Isolated follicle stimulated hormone deficiency in male: case report. BMC Res Notes. 2018;11(1):24.
- Giltay JC, et al. Apparent primary follicle-stimulating hormone deficiency is a rare cause of treatable male infertility. Fertil Steril. 1998;70(3):591-595.
- Orphanet. Isolated follicle stimulating hormone deficiency. Orpha Code 52901 (Accessed Nov 2025).
- Endocrine Society. Burger LL, et al. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33:559-584.
- Kumar N, et al. Klinefelter Syndrome – A Review. StatPearls [Internet]. 2023 Jan.
- World Health Organization (WHO). WHO Manual for Semen Analysis, 5th ed. 2010. (For general reference on interpreting azoospermia and FSH levels).
