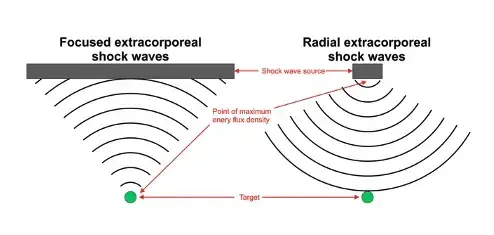
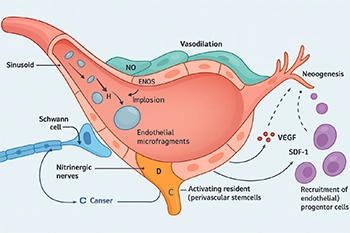
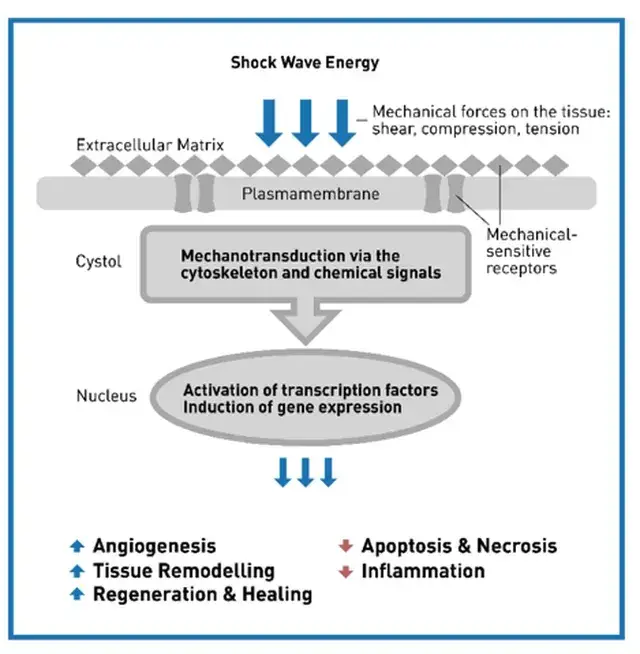
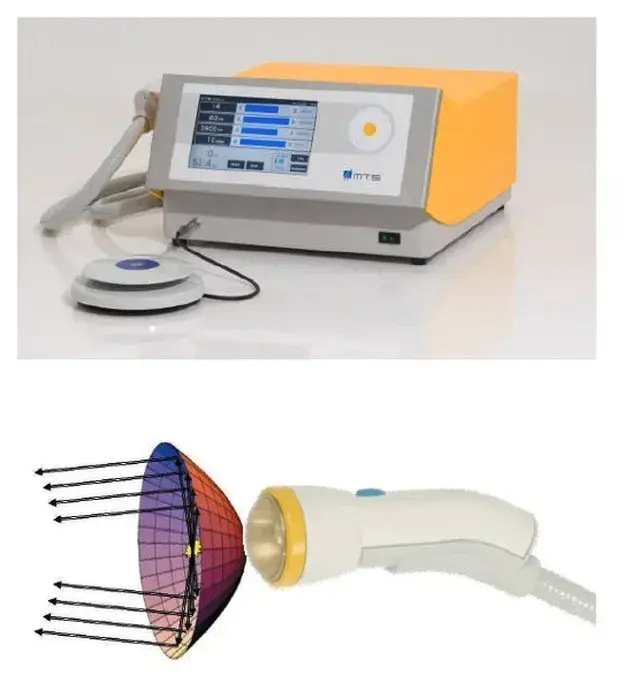
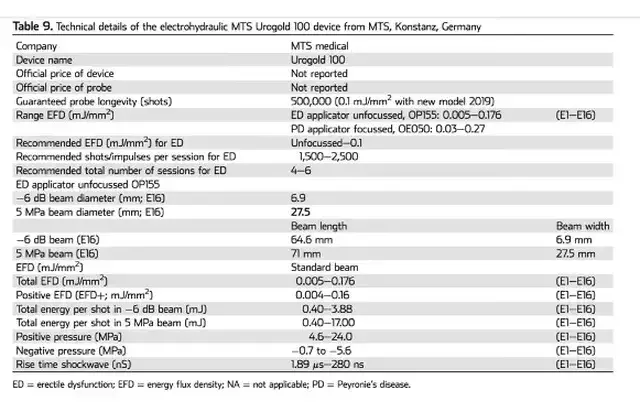
Please note: LiST presently does not have FDA clearance for use in the management of erectile dysfunction, penile curvature, penile pain, pelvic pain or low testosterone and is considered an off-label use. The device I use, the Urogold 100 MTS, has been FDA cleared for improved blood flow, pain amelioration, connective tissue activation, wound healing and is considered a device with a non-significant risk to humans. However, the device is being used ‘off-label’ for any of these men’s health issues.
1. Erectile Dysfunction
Regenerative therapies including low-intensity shockwave therapy, plasma rich plasma and stem cell therapy have generated a great deal of interest since 2015. However, low-intensity shockwave therapy has the most literature supporting its use.
20% of all men have erectile dysfunction (ED) with men of increasing age being disproportionally affected. The incidence of ED can be as high as 60% in men with Diabetes or cardiovascular disease. Although previously considered a quality-of-life issue, the presence of erectile dysfunction in an otherwise healthy man is now considered a harbinger of cardiovascular disease. Vascular factors are the leading cause of ED. A multitude of treatment options exists for those men with minimal or moderate erectile dysfunction, including pills, injections, external devices and surgical options. Unfortunately, these don’t work for every man and may interact with medications or underlying diseases. Also, they treat the symptoms and not the underlying cause of ED. LiST offers a potential treatment that can ameliorate the dysfunction and offer the potential to restore erectile function.
Several reviews of studies evaluating the use of LI-SWT have been published recently. The first was an analysis of ten randomized controlled studies completed over two years by Sokolakis and Hatzichristodoulou (Int Jour Impot Res, 31:177-194, 2019) which concluded that LiST significantly improved erectile function in patients with vascular ED. All ten studies used a validated questionnaire as the assessment tool and included men with moderate to severe ED. Their analysis also contained only 873 studies followed for less than one year. Three studies also measured penile hemodynamics.
A second systematic review by Brunckhorst et al. (Int Urol Neph, 51:773-781, 2019) reviewed eleven studies with 799 patients. Nine studies found an improvement in ED at six months after treatment with the improvement remaining above baseline out to 12 months.
A third review included seven randomized controlled studies evaluating the effect of LiST on erectile function (Dong et al., Am J Men’s Health, March-April:1-14, 2019). Evaluating outcomes by validated questionnaires (ie., International Index of Erectile Function – erectile function domain [IIEF-EF] and the Erectile Hardness Score [EHS]) they found improvement in erectile function after treatment with LiST.
Although the results have been encouraging, there are still many questions to be answered. What patients will respond to LI-SWT? Will the patients with worse erectile dysfunction, Diabetes and/or heart disease respond better or worse? Does patient age or use of oral or injectable medications used to treat erectile dysfunction affect the results of LI-SWT? What are the contraindications for the use of LI-SWT? Should we be treating men on blood thinners? How does the frequency and length of treatments affect the results? Is it better to use fewer treatments/week over a longer period of time? How does the intensity and number of shocks affect the outcome? Does the type of device matter (linear vs. focused shockwaves)? Focused shockwaves have shown their effectiveness while the results of linear shockwaves are still conflicting. When do the effects of LI-SWT occur and how long does the improvement last? These, among other questions, will need to be answered
Current literature demonstrates an improvement of erectile dysfunction with LiST. However, large, multicentered, randomized controlled studies which include not only qualitative (e.g. validated questionnaires) but also quantitative data (e.g. spectral ultrasound vascular data) are needed before LI-SWT becomes the standard of care.
2. Penile curvature and penile pain
The use of LiST for penile curvature (aka Peyronie’s disease) actually began 10 years earlier than that for treatment of erectile dysfunction. The literature for this indication is not as robust. A very nice review by Porst was recently published (Porst, H. Sex Medicine Rev 9, 93–122 (2020)). As Porst describes, the number of papers reviewed in the three published meta-analyses were limited. However, nine case-controlled studies and three randomized studies essentially came to the same conclusion that ‘LiST may be an effective and safe treatment for reducing penile plaque size and relieving pain but not for improving penile curvature’.
The design of these studies also presented a challenge on interpreting the data as it has been in the literature on ED. Porst noted that the disparity in these studies regarding the energies used, shockwave machines employed, number of shocks per session and total number of shocks given was even greater than that in the literature for ED. Obviously much more work is needed.
3. Prostatitis, Pelvic pain and Low Testosterone
There is limited literature available on the use of LiST for Prostatitis, Pelvic pain and Low Testosterone. However, this is currently an area of active investigation.
The Urogold 100 MTS is considered by the FDA to be a device with a non-significant risk to humans. In a phase 2 study (Patel et al. Sex Med, 8, 214–222, 2020) investigating the safety of LiST. They randomized patients into two Groups. Group A received once a day treatment over 5 consecutive days (Monday to Friday), in which 720 shocks of LiST were applied in every session, half to each treated region (left and right corpora cavernosa and crura). Group B consisted of once a day 3 sessions per week (Monday, Wednesday, and Friday) for 2 consecutive weeks, in which 600 shocks of LiST were applied. No immediate side effects of erythema, pain or swelling have been noted with LiST treatment for ED. Not every patient noted an improvement in erectile function. In a phase 2 trial 8% of men noted a worsening of their erectile function on a validated questionnaire (IIEF). In this same study no scar, plaque or penile deformity was noted at six to 12 months. They concluded that LiST can be used safely for the treatment of erectile dysfunction by trained and knowledgeable providers.
For questions please contact my office at 516-487-2700 or email (FrontDesk@nycryo.com)
Additional Reading:
1.Yih, J., Minton, J., Gagnon, C. & Goldstein, I. 009 Retrospective Chart Review of Treatment Outcome Following Low-Intensity Shockwave Therapy for the Treatment of Vestibulodynia with Urogold 100TM MTS. J Sex Medicine 17, S4–S5 (2020).
2.Yih, J. 195 Retrospective Chart Review of Treatment Outcome Following Low-Intensity Shockwave Therapy for the Treatment of Vestibulodynia with Urogold 100TM. J Sex Medicine 17, S67–S68 (2020).
3.Yih, J., Goldstein, S., Georgeon, L., Ramirez, R. & Goldstein, I. 318 Retrospective Review of Improvement of Erectile Function after Low Intensity Shockwave Treatment with Urogold 100TM. J Sex Medicine 17, S78 (2020).
4.Chung, E. et al. Clinical Practice Guideline Recommendation on the Use of Low Intensity Extracorporeal Shockwave Therapy and Low Intensity Pulsed Ultrasound Shockwave Therapy to Treat Erectile Dysfunction: The Asia-Pacific Society for Sexual Medicine Position Statement. World J Men’s Heal 38, (2020).
5.Yih*, J., Minton, J., Gagnon, C., Goldstein, S. & Goldstein, I. PD36-10 LUMBO-SACRAL LOW INTENSITY SHOCKWAVE THERAPY FOR GENITO-PELVIC DYSESTHESIA/PERSISTENT GENITAL AROUSAL DISORDER (GPD/PGAD) USING UROGOLD 100 MTS. J Urology 203, e726–e727 (2020).
6.Patel, P. et al. Phase II Randomized, Clinical Trial Evaluating 2 Schedules of Low-Intensity Shockwave Therapy for the Treatment of Erectile Dysfunction. Sex Med-uk 8, 214–222 (2020).
7.Porst, H. Review of the Current Status of Low Intensity Extracorporeal Shockwave Therapy (Li-ESWT) in Erectile Dysfunction (ED), Peyronie’s Disease (PD), and Sexual Rehabilitation After Radical Prostatectomy With Special Focus on Technical Aspects of the Different Marketed ESWT Devices Including Personal Experiences in 350 Patients. Sex Medicine Rev 9, 93–122 (2020).
8.Brunckhorst, O. et al. A systematic review of the long-term efficacy of low-intensity shockwave therapy for vasculogenic erectile dysfunction. Int Urol Nephrol 51, 773–781 (2019).
9.Liu, T., Shindel, A. W., Lin, G. & Lue, T. F. Cellular signaling pathways modulated by low-intensity extracorporeal shockwave therapy. Int J Impot Res 31, 170–176 (2019).
10.Sokolakis, I. & Hatzichristodoulou, G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: a systematic review and meta-analysis of randomised controlled trials. Int J Impot Res 31, 177–194 (2019).
11.Vita, R., Benvenga, S., Giammusso, B. & Vignera, S. L. Determinants of Early Response to Low-Intensity Extracorporeal Shockwaves for the Treatment of Vasculogenic Erectile Dysfunction: An Open-Label, Prospective Study. J Clin Medicine 8, 1017 (2019).
12.Dong, L. et al. Effect of Low-Intensity Extracorporeal Shockwave on the Treatment of Erectile Dysfunction: A Systematic Review and Meta-Analysis. Am J Men’s Heal 13, 1557988319846749 (2019).
13.Mauro, M. di et al. Extracorporeal Shockwave Therapy in Peyronie’s Disease: Clinical Efficacy and Safety from a Single-Arm Observational Study. World J Men’s Heal 37, (2019).
14.Mortensen, J., Ladegaard, P. B. J., Skov-Jeppesen, S. M. & Lund, L. HP-09-005 A prospective randomized placebo controlled study evaluating the combined effect of Low-intensity Extracorporeal Shockwave Therapy (LI-ESWT) and vacuum erectile device on Peyronies Disease. The Journal of Sexual Medicine 16, S47–S48 (2019).
15.Usta, M. F., Gabrielson, A. T. & Bivalacqua, T. J. Low-intensity extracorporeal shockwave therapy in the treatment of erectile dysfunction following radical prostatectomy: a critical review. Int J Impot Res 31, 231–238 (2019).
16.Wang, C.-J., Lu, Y.-M., Li, C.-C., Wu, W.-J. & Chien, T.-M. Low-intensity shockwave therapy ameliorates erectile dysfunction in men with pelvic fractures associated with urethral injury. Int J Impot Res 31, 218–222 (2019).
17.Capogrosso, P. et al. Low-Intensity Shockwave Therapy in Sexual Medicine—Clinical Recommendations from the European Society of Sexual Medicine (ESSM). J Sex Medicine 16, 1490–1505 (2019).
18.Kalyvianakis, D., Memmos, D., Mykoniatis, I., Kapoteli, P. & Hatzichristou, D. Low-Intensity Shockwave Therapy (LiST) for Erectile Dysfunction: Is It Safe for Patients on Anticoagulant Medication? J Sex Medicine(2019) doi:10.1016/j.jsxm.2019.05.008.
19.Yamaçake, K. G. R. et al. Low-intensity shockwave therapy for erectile dysfunction in kidney transplant recipients. A prospective, randomized, double blinded, sham-controlled study with evaluation by penile Doppler ultrasonography. Int J Impot Res 31, 195–203 (2019).
20.Campbell, J. D. et al. Meta-analysis of randomized controlled trials that assess the efficacy of low-intensity shockwave therapy for the treatment of erectile dysfunction. Ther Adv Urology 11, 1756287219838364 (2019).
21.Krieger, J. R., Rizk, P. J., Kohn, T. P. & Pastuszak, A. Shockwave Therapy in the Treatment of Peyronie’s Disease. Sex Medicine Rev (2019) doi:10.1016/j.sxmr.2019.02.001.
22.Katz, J. E., Clavijo, R. I., Rizk, P. & Ramasamy, R. The Basic Physics of Waves, Soundwaves, and Shockwaves for Erectile Dysfunction. Sex Medicine Rev 8, 100–105 (2019).
23.Fode, M., Russo, G. I. & Verze, P. Therapeutic areas of Li-ESWT in sexual medicine other than erectile dysfunction. Int J Impot Res 31, 223–230 (2019).
24.Mousa, R. A. et al. [50] The efficacy and safety of low-intensity shockwave therapy in erectile dysfunction. Arab J Urology 16, S25 (2018).
25.Li, P.-C. et al. [Low-intensity extracorporeal shockwave therapy for Peyronie’s disease: A preliminary study of 32 cases]. Zhonghua nan ke xue = National journal of andrology 24, 340–344 (2018).
26.Li, X. et al. Comparative effectiveness of extracorporeal shockwave, ultrasound, low-level laser therapy, noninvasive interactive neurostimulation, and pulsed radiofrequency treatment for treating plantar fasciitis. Medicine 97, e12819 (2018).
27.Zewin, T. S. et al. Efficacy and safety of low-intensity shockwave therapy in penile rehabilitation post nerve-sparing radical cystoprostatectomy: a randomized controlled trial. Int Urol Nephrol 50, 2007–2014 (2018).
28.Zou, Z., Liang, J., Liu, Z., Gao, R. & Lu, Y. Low-intensity extracorporeal shockwave therapy for erectile dysfunction after radical prostatectomy: a review of preclinical studies. Int J Impot Res 30, 1–7 (2018).
29.Man, L. & Li, G. Low-intensity Extracorporeal Shockwave Therapy for Erectile Dysfunction: A Systematic Review and Meta-analysis. Urology 119, 97–103 (2018).
30.Rizk, P. J., Krieger, J. R., Kohn, T. P. & Pastuszak, A. W. Low-Intensity Shockwave Therapy for Erectile Dysfunction. Sex Medicine Rev (2018) doi:10.1016/j.sxmr.2018.01.002.
31.Ruan, Y. et al. The effect of low‐intensity extracorporeal shockwave therapy in an obesity‐associated erectile dysfunction rat model. Bju Int 122, 133–142 (2018).
32.Fojecki, G. L., Tiessen, S. & Osther, P. J. S. Effect of Linear Low-Intensity Extracorporeal Shockwave Therapy for Erectile Dysfunction—12-Month Follow-Up of a Randomized, Double-Blinded, Sham-Controlled Study. Sex Med-uk 6, 1–7 (2017).
33.Fojecki, G. L., Tiessen, S. & Osther, P. J. S. Effect of Low-Energy Linear Shockwave Therapy on Erectile Dysfunction—A Double-Blinded, Sham-Controlled, Randomized Clinical Trial. J Sex Medicine 14, 106–112 (2017).
34.Clavijo, R. I., Kohn, T. P., Kohn, J. R. & Ramasamy, R. Effects of Low-Intensity Extracorporeal Shockwave Therapy on Erectile Dysfunction: A Systematic Review and Meta-Analysis. J Sex Medicine 14, 27 35 (2017).
35.Angulo, J. C. et al. Efficacy of low-intensity shockwave therapy for erectile dysfunction: A systematic review and meta-analysis. Actas Urológicas Españolas Engl Ed 41, 479–490 (2017).
36.Lin, G. et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. J Sex Medicine 14, 493–501 (2017).
37.Korneyev, I. A. et al. Low intensity shockwave therapy for erectile dysfunction: 6 months followup results. Urologicheskie Vedomosti 7, 5–13 (2017).
38.Inoue, S. et al. Low-intensity extracorporeal shockwave therapy for patients with severe erectile dysfunction due to radical prostatectomy. Ann Res Hosp 1, 2–2 (2017).
39.Lu, Z. et al. Low-intensity Extracorporeal Shockwave Treatment Improves Erectile Function: A Systematic Review and Meta-analysis. Eur Urol 71, 223–233 (2017).
40.Tsai, C.-C. et al. Low-Intensity Extracorporeal Shockwave Therapy Can Improve Erectile Function in Patients Who Failed to Respond to Phosphodiesterase Type 5 Inhibitors. Am J Men’s Heal 11, 1781–1790 (2017).
41.Fode, M., Lowenstein, L. & Reisman, Y. Low-Intensity Extracorporeal Shockwave Therapy in Sexual Medicine: A Questionnaire-Based Assessment of Knowledge, Clinical Practice Patterns, and Attitudes in Sexual Medicine Practitioners. Sexual Medicine 5, e94–e98 (2017).
42.Stoykov, B. A. et al. Low-Intensity Extracorporeal Shockwave Therapy – A New Approach in the Treatment of Erectile Dysfunction after Radical Prostatectomy. J Biomed Clin Res 10, 104–110 (2017).
43.Group, Y. A. U. M. H. et al. Low-intensity shockwave therapy for erectile dysfunction: is the evidence strong enough? Nat Rev Urol 14, nrurol.2017.119 (2017).
44.Kalyvianakis, D. & Hatzichristou, D. Low-Intensity Shockwave Therapy Improves Hemodynamic Parameters in Patients With Vasculogenic Erectile Dysfunction: A Triplex Ultrasonography-Based Sham-Controlled Trial. J Sex Medicine 14, 891–897 (2017).
45.Chung, E. PS-07-012 Analysis of predictors for clinical success and patient satisfaction rate following low intensity shockwave therapy in men with erectile dysfunction and Peyronie’s disease: A prospective open-label single arm study. J Sex Medicine 14, e130–e131 (2017).
46.Pan, M. M., Raees, A. & Kovac, J. R. Low-Intensity Extracorporeal Shockwave as a Novel Treatment for Erectile Dysfunction. Am J Men’s Heal 10, 146–148 (2016).
47.Kitrey, N. D. et al. Penile Low Intensity Shockwave Treatment is Able to Shift PDE5i Nonresponders to Responders: A Double-Blind, Sham Controlled Study. J Urology 195, 1550–1555 (2016).
48.Xin, Z.-C. AB067. Future ED therapy: low-energy shockwave therapy and low-intensity pulsed ultrasound therapy. Transl Androl Urology 4, AB067 (2015).
49.Olsen, A. B., Persiani, M., Boie, S., Hanna, M. & Lund, L. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol 49, 329 333 (2015).
50.Chung, E. & Cartmill, R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low‐intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: an Australian first open‐label single‐arm prospective clinical trial. Bju Int 115, 46 49 (2015).